
CAS号:25301-02-4
分子式:(C14H22O.C2H4O.CH2O)n
DMF:041031
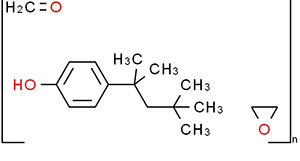
产品特性:
泰洛沙泊为非离子型表面活性剂,外观通常为无色至淡黄色的黏稠液体。它具有良好的乳化、分散和增溶性能。
技术指标(执行标准 USP41)
| Test | Specification | Test Method |
| 【Character】 | Viscous, amber liquid, having a slight, aromatic odor. May exhibit a slight turbidity. | USP41 |
| Solubility | Slowly but freely miscible in water, soluble in glacial acetic acid, in benzene, in toluene, in chloroform, in carbon tetrachloride and carbon disulfide. | USP41 |
| Refractive index(*) | 1.495~1.501 | USP41 |
| Infrared identification | Infrared absorption spectrophotometry. Meets the requirements. | USP41 |
| Residue on ignition | NMT1.0% | USP41 |
| Limit of anionic detergents | NMT0.075% | USP41 |
| Limit of ethylene oxide | NMT 10μg/g | USP41 |
| Limit of formaldehyde | NMT0.0075% | USP41 |
| Free phenol | No cloudiness or precipitation is observed immediately | USP41 |
| Absence of cationic detergents | No blue color is observed in the organic solvent layer. | USP41 |
| Cloud point | Between 92℃ and 97℃ | USP41 |
| Water content(*) | NMT 1% | USP41 |
| Toluene(*) | NMT100ppm | USP41 |
| PH(5%solution) | 4.0~7.0 | USP41 |
| Bacterial endotoxins | NMT 2EU/mg | USP41 |
| Microbial limit | TAMC: NMT 10²cfu/ml; | USP41 (2021) / (2022) |
| TYMC: NMT 10²cfu/ml; | USP41 (2021) / (2022) | |
| E. coli shall not be detected | USP41 (2021) / (2022) |
用途:
作为祛痰药,适用于痰液黏稠不易咳出的患者,可降低痰液表面张力,使痰粘度降低;也可作为药用辅料,起到增溶剂、抑菌剂的作用,用于隐形眼镜和OK镜护理液、滴眼液、滴耳液等。适用于妥布霉素滴眼液、 妥布霉素地塞米松滴眼液 、环孢素滴眼液等制剂产品
包装与储存:
本品用玻璃瓶包装,每瓶500g ,也可根据用户需要而定。密封,贮存于避光,阴凉干燥处,贮存期二十四个月。
安全防护:
本品应避免与眼睛、皮肤或衣服接触,一旦溅到身上,应立即用大量清水冲。
 苏公网安备32070302010201号
苏公网安备32070302010201号